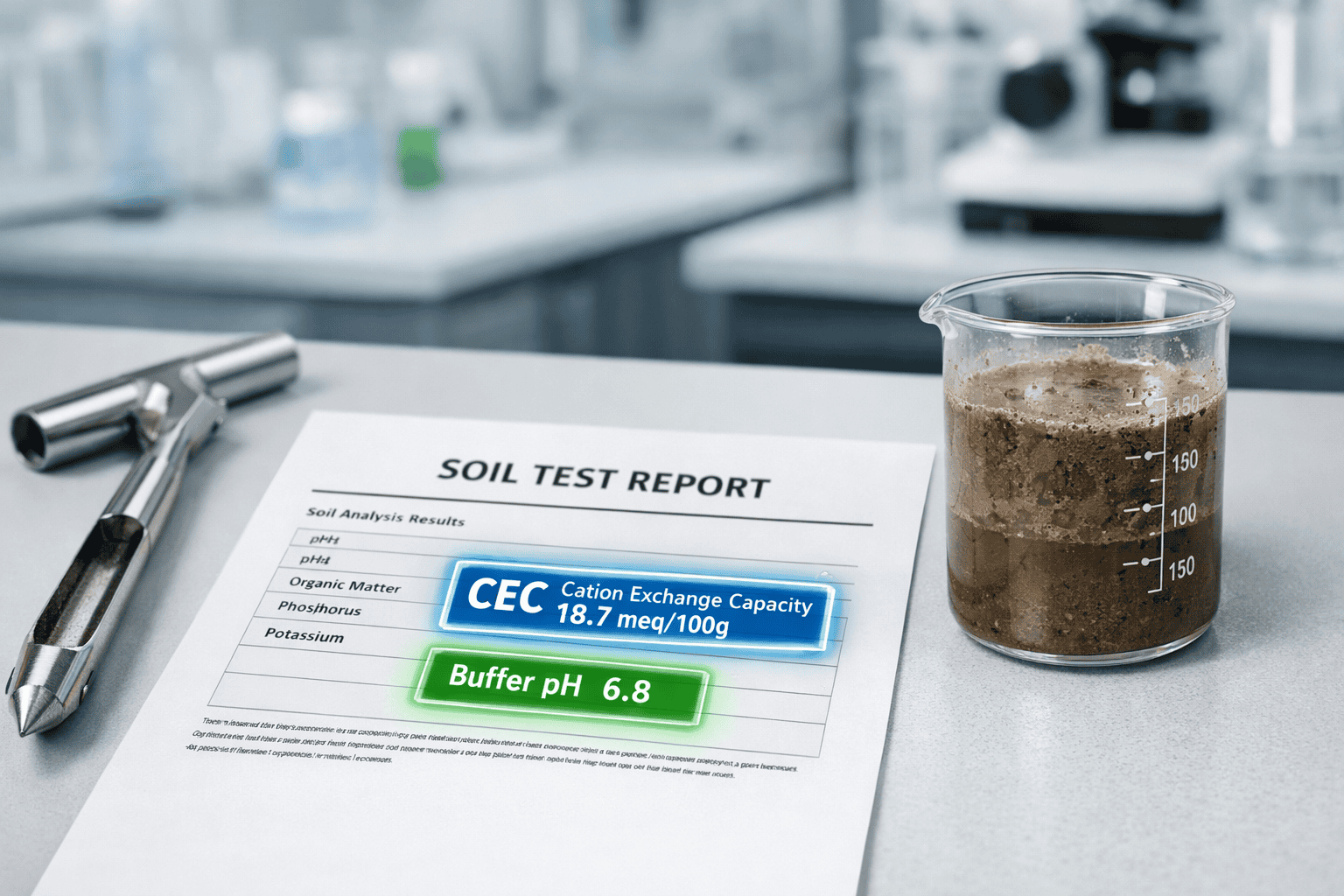
The 3-Minute Lab Report Summary
A professional soil test reveals Cation Exchange Capacity (CEC), Buffer pH, and Base Saturation—the trinity of soil chemistry that dictates nutrient availability. These metrics expose the soil’s capacity to hold nutrients, the precise lime requirement, and whether calcium-magnesium ratios support proper flocculation. Simple N-P-K numbers ignore the soil’s ability to retain amendments.
Cation Exchange Capacity (CEC): The Soil’s Fuel Tank
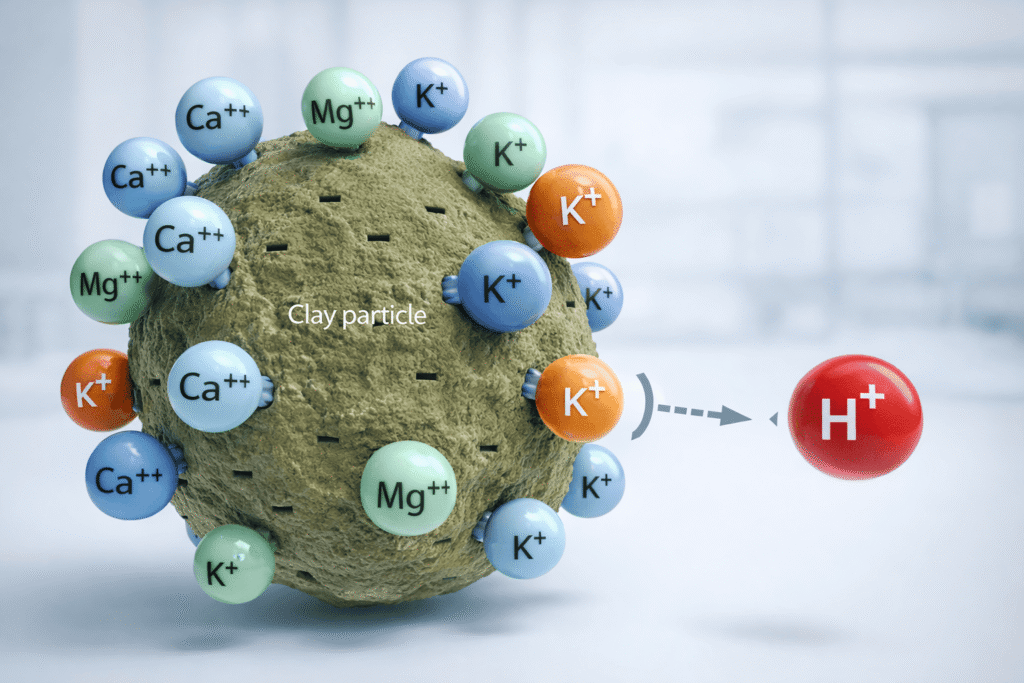
Cation Exchange Capacity measures the total number of negatively charged sites on soil colloids—primarily clay particles and humus—that can hold positively charged cations like potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺). This exchange capacity is measured in milliequivalents per 100 grams (meq/100g) or centimoles of charge per kilogram (cmol/kg).
The physics is straightforward: clay and organic matter carry negative surface charges. Nutrient cations are electrostatically attracted to these sites, preventing them from leaching through the soil profile during irrigation or rainfall. A soil with a CEC of 25 meq/100g holds approximately 2.5 times more nutrients than a sandy soil with a CEC of 10 meq/100g.
Sandy soils (CEC < 10 meq/100g) have low clay and organic matter content. They require “spoon-feeding” fertilizer—frequent, small applications—because their limited exchange sites quickly saturate and excess nutrients leach into groundwater. Clay-loam soils (CEC 15-25 meq/100g) and heavy clays (CEC > 30 meq/100g) act as nutrient reservoirs, releasing cations slowly into the rhizosphere as plant roots exchange hydrogen ions (H⁺) for adsorbed nutrients.
Cornell Soil Health Lab identifies CEC as the single most important parameter for calibrating fertilizer rates. Without knowing your soil’s CEC, you’re applying amendments blindly—either wasting money on excess fertilizer in sandy soils or under-feeding high-CEC clays.
The pH Paradox: Soil pH vs. Buffer pH
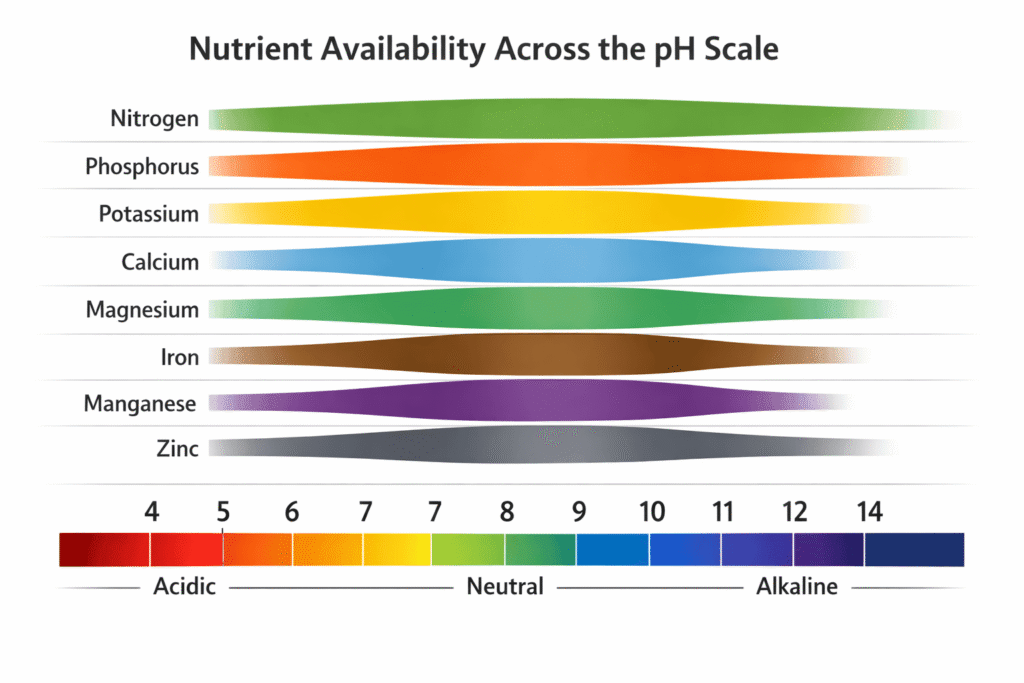
Your soil test lists two pH values: Soil pH (active acidity) and Buffer pH (reserve acidity). Understanding the distinction prevents catastrophic over-liming.
Soil pH measures hydrogen ion (H⁺) concentration in the soil solution—the water between soil particles. This is “active acidity,” determined by a 1:1 soil-to-water slurry using a standard electrode. A pH of 5.5 means the soil solution has 10 times more H⁺ ions than a neutral pH of 6.5.
Buffer pH (also called Lime Index or SMP Buffer pH) measures “reserve acidity”—the total amount of H⁺ ions held on cation exchange sites plus those dissolved in solution. Labs add a buffered solution to the soil sample, and the pH drop correlates directly to the tons of lime needed per acre to reach target pH.
Understanding your reserve acidity is vital for acid-loving crops; follow our Potato Fertilizer Protocol to see how specific pH levels prevent Common Scab development.
Here’s the critical point: Soil pH alone does not determine lime requirement. A sandy soil (low CEC) at pH 5.5 might need 1 ton of lime per acre, while a clay soil (high CEC) at the same pH 5.5 could require 4 tons. The clay’s exchange sites hold massive reserves of H⁺ that must be neutralized.
Texas A&M AgriLife Extension emphasizes that Buffer pH is the only valid metric for calculating lime applications. Applying lime based solely on Soil pH results in either insufficient correction (acidic pockets remain) or aluminum toxicity from over-liming sandy soils.
Practical interpretation: If your Soil pH is 5.8 and Buffer pH is 6.4, you need approximately 2-3 tons of agricultural lime (calcium carbonate) per acre to reach pH 6.5. If Buffer pH is 6.8, you need less than 1 ton.
Base Saturation (The Expert Ratio)
Base Saturation expresses the percentage of CEC occupied by specific base cations (calcium, magnesium, potassium, sodium) versus acidic cations (hydrogen, aluminum). This is where soil chemistry transitions from basic fertility to precision agronomics.
The Albrecht System, developed by William Albrecht at the University of Missouri, defines optimal Base Cation Saturation Ratios (BCSR) for agricultural soils:
- Calcium (Ca²⁺): 60-70% of CEC
- Magnesium (Mg²⁺): 10-20% of CEC
- Potassium (K⁺): 2-5% of CEC
- Sodium (Na⁺): <1% of CEC
- Hydrogen (H⁺) + Aluminum (Al³⁺): 10-20% of CEC (depending on target pH)
These ratios aren’t arbitrary. Calcium promotes soil aggregation and flocculation—the clumping of clay particles into larger, stable crumbs that improve water infiltration and root penetration. Magnesium plays a secondary structural role but excess Mg²⁺ (>25% saturation) causes dispersion, where clay particles repel each other, creating compacted, waterlogged soils.
Example calculation: Your lab report shows:
- CEC: 20 meq/100g
- Ca²⁺: 10 meq/100g (50% saturation)
- Mg²⁺: 5 meq/100g (25% saturation)
- K⁺: 0.8 meq/100g (4% saturation)
Interpretation: Calcium saturation is deficient (target 60-70%). Magnesium is excessive (target 10-20%), likely causing poor soil structure and slow drainage.The corrective strategy: If pH is low, apply high-calcium lime (calcium carbonate) to increase Calcium saturation while raising pH. If pH is already at the target level but Magnesium is excessive, apply gypsum (calcium sulfate); the sulfate ions will bind with the displaced magnesium for leaching, allowing Calcium to occupy the exchange sites without altering the soil pH.
Cornell’s data shows that maintaining Ca:Mg ratios between 3:1 and 7:1 optimizes microbial activity in the rhizosphere. Ratios below 2:1 suppress beneficial bacteria and increase susceptibility to root pathogens.
The W-Pattern Sampling Protocol
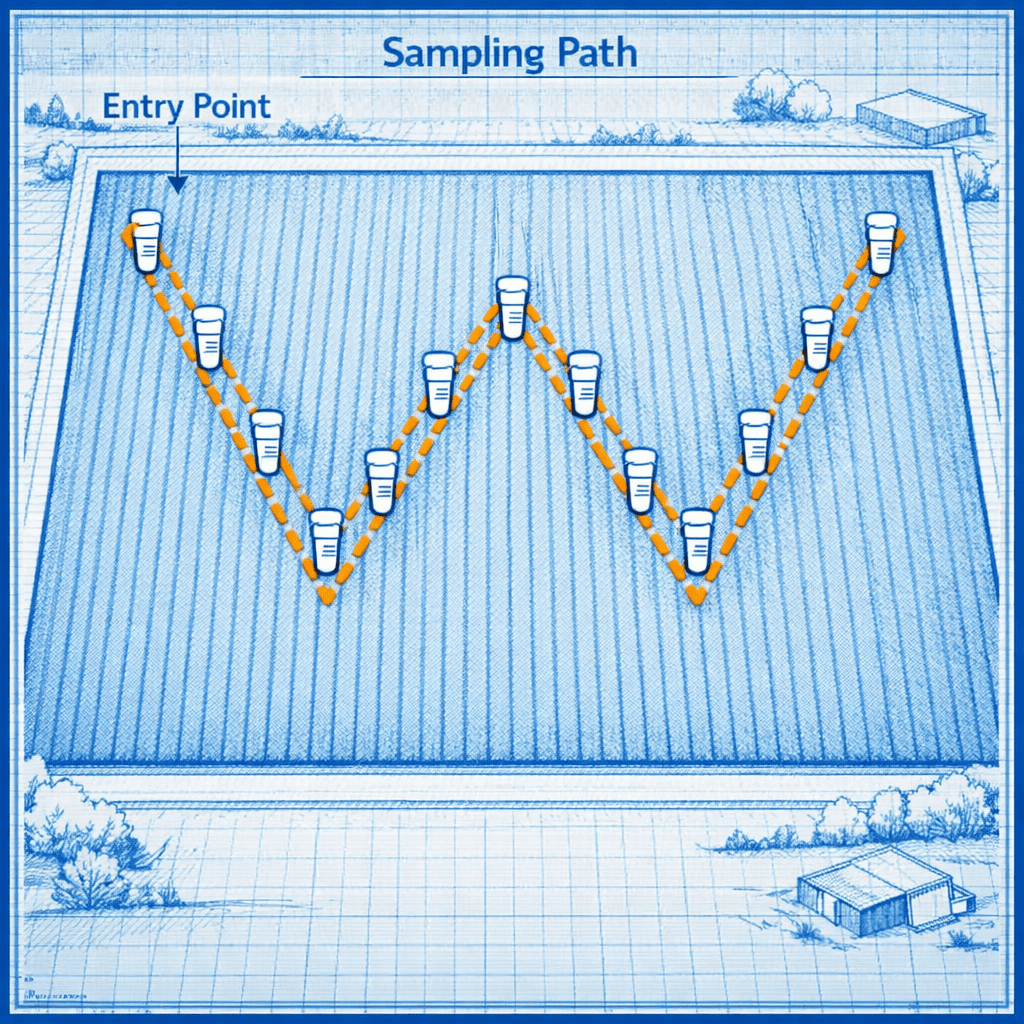
Laboratory accuracy depends entirely on submitting a representative sample. One random core cannot characterize a heterogeneous field.
Equipment required:
- Stainless steel soil probe or auger (chrome-plated to prevent metal contamination)
- Clean plastic bucket (avoid galvanized metal—zinc contamination skews micronutrient results)
- Zipper-lock plastic bags (1-pint capacity)
Step-by-step W-Pattern protocol:
Divide the sampling area. For uniform fields, sample 1 core per acre. For gardens or variable terrain, divide into management zones based on topography, crop history, or soil color.
Walk a W-pattern. Enter the field at one corner and walk diagonally, sampling at 10-15 evenly spaced points along the W path. This captures spatial variability better than random sampling.
Sample depth: For annual crops and lawns, collect cores from 0-6 inches (plow layer). For orchards and perennials, sample 0-12 inches. Remove surface debris and thatch before probing.
Composite the cores. Place all cores in the bucket, mix thoroughly, and remove approximately 2 cups (400-500g) for submission. Break up clumps but do not pulverize—soil structure affects bulk density measurements.
Avoid contamination. Never sample immediately after fertilizer application, near roads (vehicle exhaust deposits lead), or in manure piles. Wait 6-8 weeks post-amendment for accurate results.
Timing matters. Sample during the dormant season (fall or early spring) when microbial activity stabilizes. Mid-summer sampling during active crop uptake produces artificially low nutrient readings.
If your soil test results don’t align with crop appearance, check our diagnostic guide on identifying Nitrogen deficiency to confirm if leaching has occurred post-sampling
Pro tip: Label each sample with GPS coordinates or field identifiers. Texas A&M recommends archiving soil test reports to track nutrient trends over 5-10 year cycles, revealing whether your amendment strategy improves or degrades soil health.
Professional Lab vs. Home Test Kit
| Testing Method | Accuracy Level | Data Provided | Recommendation |
|---|---|---|---|
| Professional Lab (Mehlich-3 Extraction) | ±5% error; ISO 17025 certified | pH, Buffer pH, CEC, Base Saturation (Ca, Mg, K, Na), Micronutrients (Fe, Mn, Zn, Cu, B), Organic Matter %, Texture Analysis | Required for precision agriculture, orchards, commercial greenhouses. Retest every 3 years. |
| Home pH Probe (Handheld Electrode) | ±0.5 pH units; drift after 50 uses | Soil pH only (active acidity) | Useful for monitoring pH trends between lab tests. Calibrate weekly with pH 4.0 and 7.0 buffers. |
| Colorimetric Test Kit (Litmus/Reagent) | ±1.0 pH units; ±50% for NPK | Approximate pH, N-P-K (color-matched charts) | Acceptable for hobbyists. Cannot determine CEC, Buffer pH, or Base Saturation. |
| Electrical Conductivity (EC) Meter | ±2% in 0-5,000 µS/cm range | Total dissolved salts (measured in microsiemens per centimeter) | Essential for greenhouse substrates and saline soils. Pair with professional test for ion-specific analysis. |
⚠️ Technical Note on Phosphorus: In calcareous or alkaline soils (pH >7.5), standard Bray P1 or Mehlich-3 extractions can be neutralized by calcium carbonate, producing false-low readings. Ensure your lab uses Olsen P (Sodium Bicarbonate) extraction for accurate phosphorus data in high-pH environments.”
Key insight: Handheld probes measure only the soil solution (active acidity). They miss reserve acidity, leading to chronic under-liming. Professional labs use buffered extractions (Mehlich-3, Bray-P1) that chemically simulate long-term nutrient availability in the rhizosphere.
Advanced Interpretation: Reading Between the Numbers
The Nitrogen Mobility Warning: Most soil tests provide a ‘Nitrate-N’ snapshot, which represents only what is available in the soil solution at that exact moment. Because nitrogen is highly mobile and subject to leaching or volatilization, these numbers are not reliable for long-term planning. Use Pre-Sidedress Nitrate Tests (PSNT) during the active growing season to verify actual demand.
Beyond individual parameters, expert interpretation identifies antagonistic relationships between nutrients:
Excess potassium (K⁺) blocks magnesium uptake. If K saturation exceeds 7%, plants show Mg deficiency symptoms (interveinal chlorosis) even when Mg levels appear adequate. The solution: reduce potash applications and apply magnesium sulfate (Epsom salt) as a foliar spray to bypass root competition.
High phosphorus (P) precipitates zinc (Zn). Phosphate ions (PO₄³⁻) bind Zn²⁺ in insoluble compounds. If Mehlich-3 phosphorus exceeds 100 ppm and Zn tests below 3 ppm, apply chelated zinc (Zn-EDTA) to restore balance.
Micronutrient balance is especially critical for fruit set; our Olive Tree Fertilizer Guide details how Boron and Zinc interact during the inflorescence stage
Sodium (Na⁺) saturation above 3% signals sodic soil. Sodium disperses clay particles, destroying soil structure and creating impermeable layers. Treatment requires gypsum applications (2-5 tons per acre) to displace Na⁺ with Ca²⁺, followed by deep tillage to break up compaction.
Organic matter (OM) correlates with CEC. Each 1% increase in OM adds approximately 2-3 meq/100g of CEC. Texas A&M data shows that building OM from 2% to 5% through cover cropping and compost amendments increases CEC by 6-9 meq/100g—equivalent to adding 15-20% clay content without altering soil texture.
When to Retest: Timing Your Soil Audits
Baseline test: Conduct a comprehensive analysis before establishing perennial crops, new lawns, or converting land use. This documents initial fertility and guides the first 2-3 years of amendments.
Maintenance testing: For annual vegetable gardens and row crops, retest every 2-3 years. For established orchards and perennial pastures, test every 3-5 years. High-intensity greenhouse production requires annual testing due to rapid nutrient depletion.
Post-amendment verification: After applying major lime or gypsum treatments, wait 6 months for reactions to complete, then retest to verify pH and Base Saturation corrections.
Seasonal considerations: Fall sampling (September-November) captures nutrient levels after crop uptake ceases but before winter leaching. Spring sampling (March-April) reveals overwinter losses and guides in-season fertilizer sidedressing.
Conclusion: From Data to Decision
Reading a soil test transforms empirical guesswork into evidence-based fertility management. The hierarchy is clear: CEC defines your soil’s nutrient-holding capacity. Buffer pH calculates precise lime requirements. Base Saturation optimizes structural stability and microbial function.
Ignore home test kits that promise instant results with colorimetric cards. Invest $30-50 in a professional lab analysis that provides Mehlich-3 extractable nutrients, complete cation exchange data, and agronomic recommendations calibrated to your region’s crop response curves.
The 2026 protocol demands precision. Your soil is not generic “dirt”—it’s a dynamic cation exchange matrix where chemistry, physics, and biology intersect. Master these metrics, and you control the most fundamental variable in agricultural productivity.